FDA recognizes Nistec as medical devices manufacturer
The US Food and Drug Administration (FDA) has recognized the Nistec Merkaz electronics plant in Petah Tikva as an authorized manufacturer of medical devices.
The registration was achieved based on the experience garnered by Nistec from its collaboration with its customer RenalSense of Jerusalem, who are internationally commercializing a digital monitoring system for kidney treatment.
The FDA is the US federal body that supervises and regulates food and medications for humans and animals, cosmetics, medical devices, blood products, tobacco products, and devices that emit electromagnetic waves, The FDA has substantial global influence and its decisions carry great weight in the product approval process in many countries. The FDA’s approval process for medications and medical devices is long and meticulous.
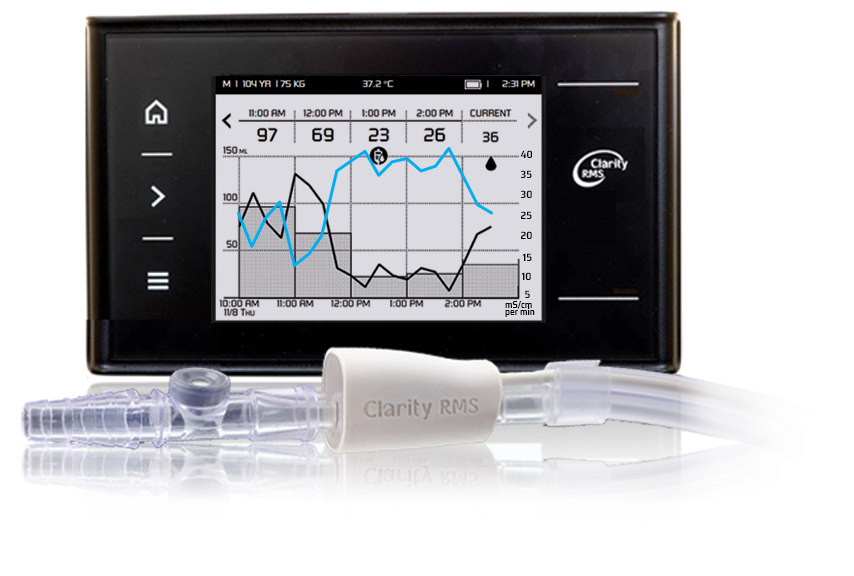
The FDA has recognized the Nistec electronics plant in Petah Tikva as an authorized manufacturer of medical devices. The company obtained authorization to manufacture innovative products, like Clarity RMS developed by RenalSense of Jerusalem.
RenalSense’s Clarity RMS system continuously monitors urine flow automatically displaying and transmitting real-time information essential for clinicians. This information, which reflects changes in kidney function, provides an early indicator of kidney damage, enhancing prevention and appropriate treatment of Critical Care patients..
Nistec carries out full production of the systems, including the electronic board, the mechanical assembly, the electrical testing, and packaging through delivery to the end-customer.
FDA registration will help Nistec win other customers seeking to market their products under the FDA.
“We have worked with RenalSense for ten years, since the early product development stages and through the FDA registration for the product, which the company obtained several years ago.” says Elad Nissan, plant manager of Nistec Merkaz. “We are very proud that the FDA has recognized Nistec’s capability to produce “Made in Israel” products that brings relief to many people all over the world.”
“Our collaboration with Nistec’s experienced team, has been a key factor to delivering an outstand product. And Nistec management has been extremely helpful and supportive from day one”, said Moti Barda, RenalSense COO.

